Animal Health Clinical Trials
Introduction to Animal Health Clinical Trials
Animal health clinical trials are a crucial aspect of advancing veterinary medicine, ensuring the development of safe and effective treatments for animals. These trials are designed to assess the efficacy and safety of new veterinary drugs, devices, and procedures, mirroring the process used in human clinical trials. The primary goal is to improve animal health and welfare, which in turn can have significant impacts on human health, especially considering the role animals play in food production and as companions.
Importance of Animal Health Clinical Trials

The importance of animal health clinical trials cannot be overstated. They provide a controlled environment where new treatments can be tested, ensuring that any intervention is both safe for the animal and effective in treating or preventing disease. This not only benefits the animals directly but also has broader implications: - Public Health: By ensuring the health of animals used in food production, the risk of zoonotic diseases (diseases that can be transmitted from animals to humans) is reduced. - Economic Benefits: Healthy animals are more productive, leading to better agricultural outputs and economic benefits for farmers and the industry as a whole. - Companion Animals: For pet owners, successful clinical trials mean access to better treatments for their pets, improving the quality of life for both the animals and their owners.
Phases of Animal Health Clinical Trials
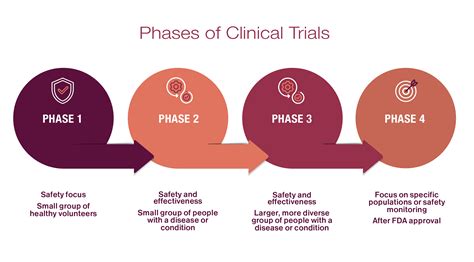
Similar to human clinical trials, animal health clinical trials are conducted in phases, each designed to answer specific questions about the treatment being tested: - Phase 1: Focuses on the safety of the treatment, often involving a small number of animals to assess tolerance and potential side effects. - Phase 2: Evaluates the efficacy of the treatment, determining the appropriate dosage and further assessing safety in a larger group of animals. - Phase 3: Involves larger, more diverse animal populations to confirm efficacy, monitor side effects, compare the treatment to commonly used treatments, and collect information that will allow the experimental treatment to be used safely. - Phase 4: Post-marketing studies to gather information on the treatment’s effect in various populations and any side effects associated with long-term use.
Challenges in Conducting Animal Health Clinical Trials
Conducting animal health clinical trials poses several challenges: - Regulatory Compliance: Ensuring trials meet regulatory requirements, which can vary significantly between countries. - Ethical Considerations: Balancing the need for animal testing with ethical concerns, ensuring animal welfare is protected. - Funding: Securing funding for trials, which can be costly and time-consuming. - Variability: Managing variability in animal populations, which can affect trial outcomes.
Role of Veterinarians and Researchers

Veterinarians and researchers play pivotal roles in animal health clinical trials. They are responsible for: - Designing Trials: Developing trial protocols that are both scientifically robust and ethically sound. - Conducting Trials: Overseeing the execution of trials, ensuring adherence to protocols and the welfare of animals involved. - Analyzing Data: Interpreting results to determine the efficacy and safety of treatments. - Publishing Findings: Sharing results with the scientific community to advance veterinary medicine.
Technological Advancements

Technological advancements are transforming the field of animal health clinical trials, offering new tools and methodologies: - Digital Health Technologies: Utilizing wearable devices, mobile apps, and telemedicine to enhance trial design, data collection, and participant engagement. - Genomics and Precision Medicine: Tailoring treatments to individual animals based on genetic profiles, potentially leading to more effective and targeted therapies. - Artificial Intelligence (AI) and Machine Learning (ML): Applying AI and ML to analyze large datasets, predict outcomes, and identify new targets for treatment.
Ensuring Animal Welfare

Ensuring animal welfare is a cornerstone of animal health clinical trials. This involves: - Minimizing Pain and Distress: Implementing protocols to minimize animal suffering. - Providing Appropriate Care: Ensuring animals receive appropriate care and housing during trials. - Monitoring Welfare: Continuously monitoring animal welfare and adjusting trial protocols as necessary.
🐾 Note: Ensuring the welfare of animals in clinical trials is not only ethical but also crucial for obtaining reliable and meaningful results, as stressed animals may respond differently to treatments.
Future Directions

The future of animal health clinical trials looks promising, with ongoing advancements in technology, a growing understanding of animal diseases, and an increased focus on animal welfare. As the field continues to evolve, we can expect to see: - Increased Collaboration: Greater collaboration between researchers, veterinarians, and industries to advance veterinary medicine. - Personalized Medicine: More treatments tailored to individual animals or specific conditions. - Alternative Models: Exploration of alternative models to animal testing, such as in vitro studies and computer simulations.
In summary, animal health clinical trials are essential for the development of new treatments and the advancement of veterinary care. By understanding the importance, phases, challenges, and future directions of these trials, we can work towards improving animal health and welfare, which has far-reaching benefits for both animals and humans. This continuous effort and advancement in the field will lead to better health outcomes for animals and contribute significantly to public health, food safety, and the overall well-being of society.
What are animal health clinical trials?

+
Animal health clinical trials are research studies designed to assess the safety and efficacy of new veterinary drugs, devices, and procedures.
Why are animal health clinical trials important?

+
They are crucial for advancing veterinary medicine, ensuring public health, and improving animal welfare, which has economic and social benefits.
What phases do animal health clinical trials go through?

+
Animal health clinical trials go through phases similar to human trials, including Phase 1 for safety, Phase 2 for efficacy and side effects, Phase 3 for larger scale efficacy and safety, and Phase 4 for post-marketing surveillance.
Related Terms:
- Veterinary clinical trials database
- AVMA clinical trials
- Veterinary clinical trials phases
- Animal clinical trials
- Clinical trials for dogs
- Veterinary clinical research jobs